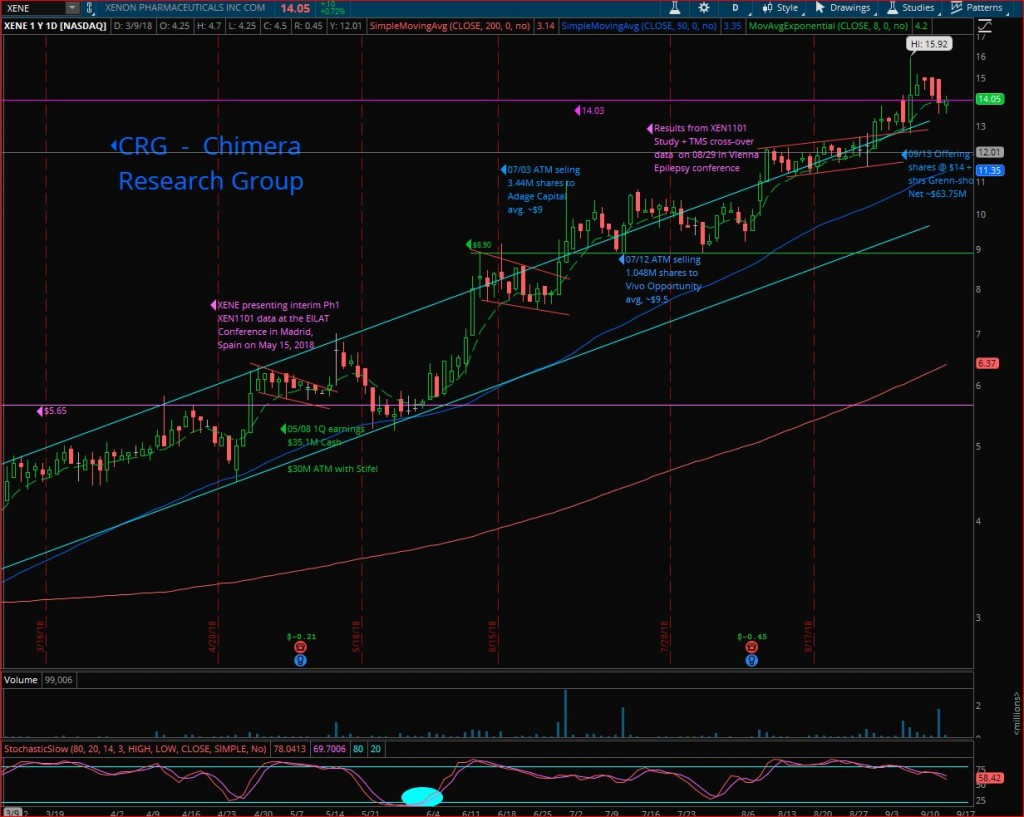
Last week, Xenon pharmaceuticals (XENE) passed one of the most important tests in the stock market, where the share price closed on Friday at $14.05, above the $14.03 resistance line, despite the news of the $50 million public offering, followed by the news of upsizing the offering to $63 million at $14.00 per share, only a 1.8% discount relative to its Wednesday closing price of $14.25. The upsizing of the offering shows the strong demand from institutional buyers, willing to start a new position or adding to their position at $14.00, despite the huge run from a $2.10 low back in November 2017 to the $14’s area. As we know, the institutions who participated in the financing are not building a position for a daily or monthly trade, but for a mid to long term holding. I suspect they see Xenon as having near term potential of achieving $20 to $25, assuming continued positive data and news flow, and with the medium / long term potential of becoming much bigger – a CNS power house.
With the recent financing, XENE is very well positioned to advance its pipeline of epilepsy assets, but maybe it is the only non-epilepsy asset in the pipeline that stands to benefit the most from xenon’s improved balance sheet.
Xenon’s pipeline has undergone an incredible transformation over the past 12-months. The company now has two Phase 2 ready epilepsy assets, (XEN901 and XEN1101) and has recently added a Phase 3 orphan pediatric opportunity to its pipeline with XEN496 (ezogabine). However, there is another Phase 2 asset on their pipeline chart, that really goes unnoticed, flunarizine or XEN007, that might warrant a closer look. Frankly, the company has been very quiet on XEN007, and I guess purposefully so, given the focus on XEN1101 and XEN901 data and the addition of a late stage program with XEN496. But it was in my reading of the latest Jefferies note on XENE, and their comment that they believe XEN007 is “underappreciated by the Street”, that I thought it timely to take a closer look at XEN007.
XEN007 for Hemiplegic Migraine (HM)
Developed by Janssen, XEN007 is a calcium channel antagonist (Cav2.1), that has been approved in many jurisdictions outside the U.S. (including most of Europe) for the treatment of chronic migraine. The drug has never been approved in the U.S. It was first announced as a pipeline asset for Xenon in March 2018. At that time the company stated that it would focus XEN007’s development on a rare form of migraine known as hemiplegic migraine (HM) that afflicts approximately 60,000 Americans. Like its other assets in development, Xenon believes there is a strong genetic rationale for developing XEN007 for HM and there are no CNS acting calcium channel blocker approved in the US. Over activity, or gain-of-function, in the channel Cav2,1 is thought to be a cause of HM. Given its mechanism of action, flunarizine, is frequently used in Europe for managing HM, even though it is not approved for this indication. In the U.S., where flunarizine is unavailable, physicians frequently use the cardiac targeted calcium channel blocker, verapamil, to treat HM. So clearly there is awareness among physicians treating HM in the U.S. of the role calcium channels play in the illness, they simply do not have a CNS targeted calcium channel blocker available. Sounds like a great opportunity to me.
Xenon has already received orphan drug designation for XEN007 in HM, and this would be their main source of market protection, given that the product is generic ex-US. The company did state in their March conference call that they were also pursuing other IP strategies (one has to assume formulation or use patents) to add further protection to the asset.
Since their disclosure of XEN007 as a pipeline product in March, the company has been very quiet on the asset. Who can blame them, they have been very busy with their epilepsy pipeline. Nevertheless, I found it interesting that Jefferies in their note last week, decided to spend some time on XEN007. In particular, the analyst referenced the parallels between the development path for XEN496 and what will likely be pursued or XEN007. Xenon has already commented that they have exclusive access to historical flunarizine regulatory and clinical data for the purposes of interacting with FDA on XEN007, much like they have with GSK for XEN496. This should allow Xenon to forego much of the preclinical and early clinical work for XEN007 and likely allow them to move straight into efficacy studies.
As to when an efficacy study could start; the company has stated previously that XEN007 could be in the clinic as early as 2018. During their recent BioCentury presentation (Link to the presentation) XENE’s CEO stated they could have XEN007 in “…one or more Phase 2 studies in the near term.” Building on that, the company not only mentioned their ongoing interest in taking XEN007 into Phase 2 for HM, but also potentially into “a number” of investigator sponsored studies. The company described these investigators sponsored studies as being small, 50-60 patient, studies with potential data readouts in 2019.
If XEN007 were to be tested in a few smaller studies this could fill a nice data void for Xenon. The company had a busy year for data in 2018, but 2019 is likely to be a year of more trial initiations than data readouts. We are anticipating study starts for all three clinical assets, XEN901, XEN1101, and XEN496 over the next 3-9 months, with the earliest possible data readout being late 2019. So, if there were a couple smaller studies with XEN007 with data readouts mid-2019, that could satiate data-hungry investors until the bigger readouts later in the year 2019.
Regardless, XEN007 looks like another well thought out product candidate, with an above average chance of success. It complements the company’s ion channel and CNS focus, and should be able to move straight into efficacy studies in the near term. Hopefully we get an update on XEN007 shortly.
Disclosure: Author is Long XENE













