This is a quick take on the TGTX efficacy data presented at ASH this past weekend. TG Therapeutics reported PK data as well as results from the higher 800 mg cohort. It appears this step up from 400 mg has finally resulted in some activity and the company continues to dose escalate; an 1800 mg cohort is now open.
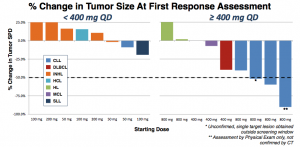
Looking at the waterfall chart, the difference between 400 mg or less and 800 mg is quite apparent.
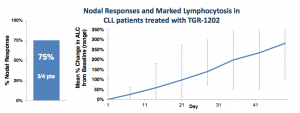
Five CLL patients were enrolled at the 800 mg dose, one was removed due to an unrelated Richter’s transformation. Of the remaining four, three achieved a nodal response (1 unconfirmed). At first blush, this looks pretty good, and approximates rates seen in other BCR pathway inhibitors. Still, some questions remain.
TGR-1202 is a potent inhibitor with a long half-life. Interestingly, while 200 mg gives a plasma concentration well above the IC90 for PI3K delta inhibition, there is no activity at that level. A 4-fold dose increase is required before any responses are seen.
As with Idelalisib, which TG Therapeutics frequently compares its compound to, TGR-1202 induces lymphocytosis, as shown below:
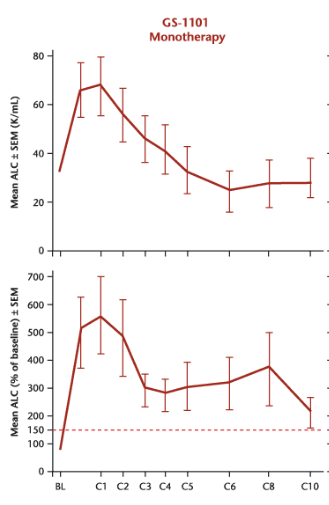
But this is a very odd graph. Lymphocytosis occurs when drugs such as Idelalisib force CLL cells out of their sanctuaries and into circulation. This is typically a fairly rapid event which peaks after several weeks then subsides. Below are data from an Idelalisib (GS-1101) phase I/II study with patients treated in 28-day cycles.
So while TGR-1202 also induces lymphocytosis, the kinetics appear slower than that of other PI3K delta inhibitors. Either 800 mg is still far too low a dose or the drug behaves differently from others in its class.
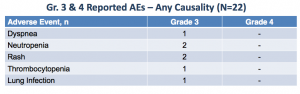
At this point, TGR-1202 appears to have a relatively tame safety profile:
In all likelihood, this is due to the sub-optimal dosing received by patients thus far, along with short durations on treatment. Adverse effects are almost certain to increase with recruitment into higher dose cohorts.
We now know why TG Therapeutics is so focused on a combination approach for their PI3K delta inhibitor: activity is seen only at very high concentrations and the compound fails to clear lymphocytosis. We know from clinical trials of Idelalisib that combinations with agents such as Rituximab or bendamustine significantly improve the drug’s performance. Such combinations also lead to rapid decrease of lymphocytosis, leading to higher conversion rates of nodal responses to Partial response.
There is little that is compelling in the TGR-1202 data. Management continues to keep the compound’s structure a secret, however, it is doubtful many others are looking to imitate.
Disclosure: Long GILD